
The Contract Service Unit of
APR Applied Pharma Research SA
APR Applied Pharma Research SA
APR LAB is a fully integrated Bio-pharma contract development and analytical service provider.
Our mission is turn Client strategic vision into Projects and Products, widening the pipeline and the portfolio' value proposition.
Our mission is turn Client strategic vision into Projects and Products, widening the pipeline and the portfolio' value proposition.

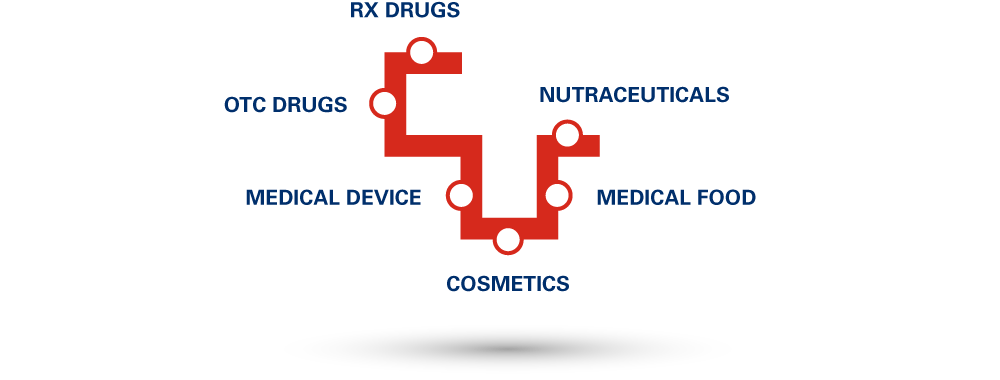
30+ years of R&D experience in developing innovative products. Technical and analytical know-how combined with regulatory proficiency covering conventional and unconventional solid, non-solid and liquid dosage forms across multiple Healthcare Categories: Pharmaceutical, Medical Device, FSMP, Medical Food, Nutraceutical, Cosmetics.

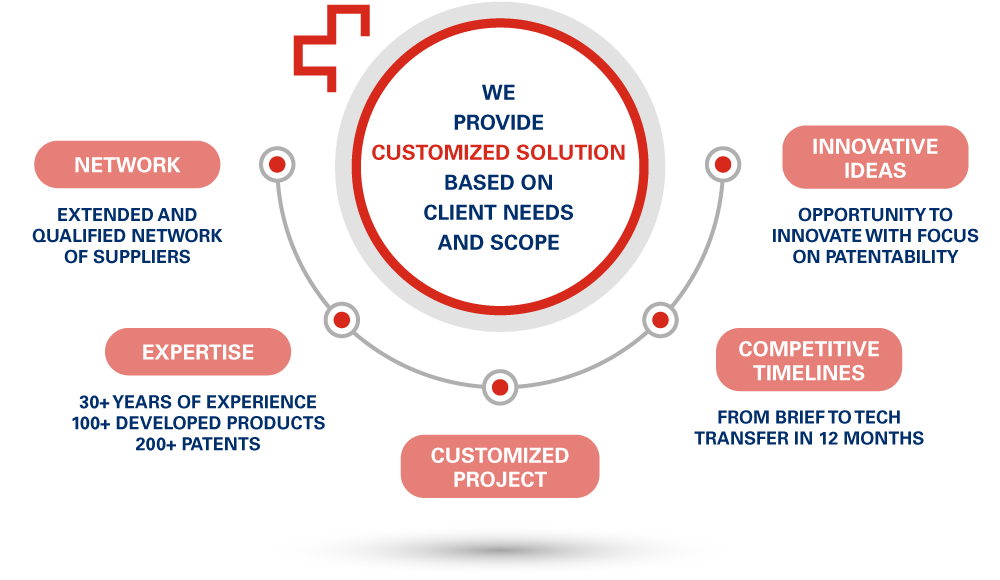
We have in house all the competencies to provide a comprehensive, scientifically driven risk-benefit analysis of your project. Integrate regulatory requirements and investment information to manage all phases of product development to minimize times and costs.
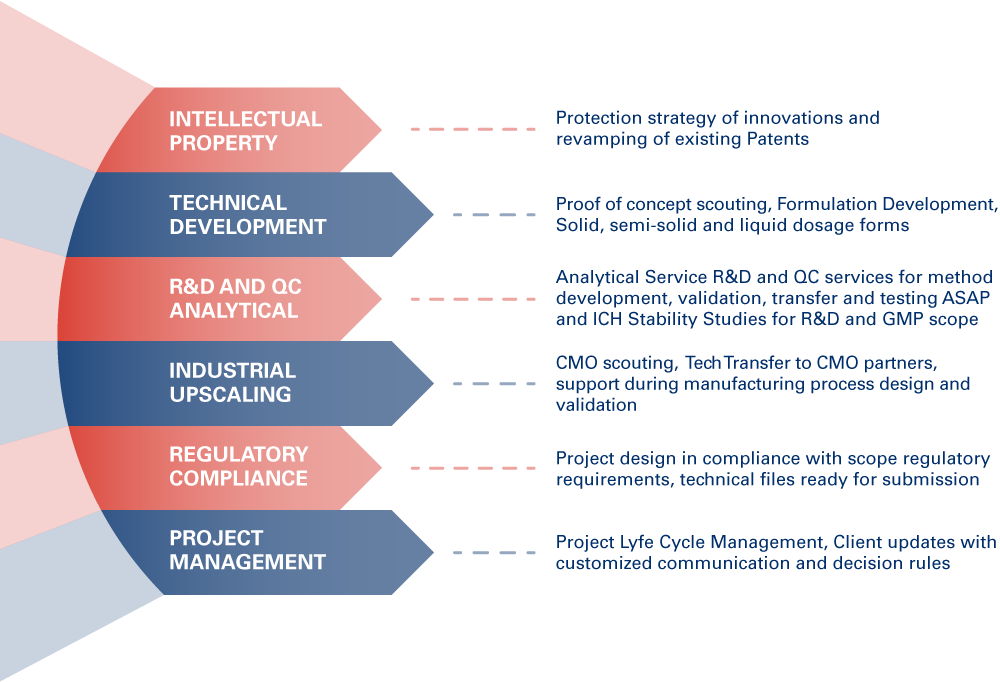
We can develop new products, new delivery, repurpose, assess products continuous improvement and compliance with new Markets.
We are a lean and flexible in-house team of professionals with deep industry experience coupled with an extensive qualified network of clinical advisors and key opinion leaders, contract research and manufacturing organization, regulatory consultants.
We are a lean and flexible in-house team of professionals with deep industry experience coupled with an extensive qualified network of clinical advisors and key opinion leaders, contract research and manufacturing organization, regulatory consultants.

APR LAB manages all aspects of product development providing comprehensive development services, from product design to formulation, generating new IPs or revitalizing old patents, offering lean manufacturing of lab scale batches for pre-clinical studies and a qualified network of CMOs to rapidly scale from clinical and pilot programs to commercial ones, with our state of the art laboratories we manage a broad range of analytical services including method development, validation and transfer, R&D and QC testing, ICH and GMP stability programs.

A site with 800 m2 in-house state of the art galenical and analytical laboratories let us perform formulation trials of solid, semi-solid and liquid dosage forms, technical development and set up of new analytical methods.
We can manufacture and package small scale non-GMP batches for prototypes performance and preclinical studies. We can manage GMP analytical validation, transfer and testing and ICH stability programs.
We can manufacture and package small scale non-GMP batches for prototypes performance and preclinical studies. We can manage GMP analytical validation, transfer and testing and ICH stability programs.






